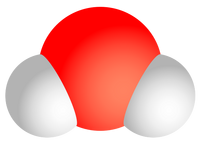
This represents a basic water molecule. There are two hydrogen atoms (white), and one oxygen atom (red).
Water is a common chemical substance that is essential for the survival of all known forms of life. In typical usage, water refers only to its liquid form or state, but the substance also has a solid state, ice, and a gaseous state, water vapor or steam. Water covers 71% of the Earth's surface. On Earth, it is found mostly in oceans and other large water bodies, with 1.6% of water below ground in aquifers and 0.001% in the air as vapor, clouds (formed of solid and liquid water particles suspended in air), and precipitation. Saltwater oceans hold 97% of surface water, glaciers and polar ice caps 2.4%, and other land surface water such as rivers, lakes and ponds 0.6%.
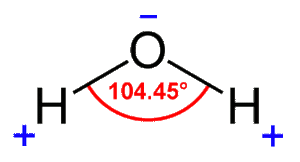
This is a diagram showing the atoms needed for a water molecule. The Chemical bonds holding this together form an exactly 104.5º interior angle.
A very small amount of the Earth's water is contained within biological bodies and manufactured products. Other water is trapped in ice caps, glaciers, aquifers, or in lakes, sometimes providing fresh water for life on land.
Pure water is actually six different substances, depending on which isotopes of both O and H go into a molecule. The resulting mass differences are enough to markedly change the behavior of water. For example, the melting point of deuterium oxide (heavy water) is 3.8 C.

|
The original article was at Water. The list of authors can be seen in the page history. As with Chemistry, the text of Wikipedia is available under the GNU Free Documentation License. |